The Asymmetric Brain
Structural and functional asymmetries in the nervous system are found throughout the animal kingdom. In humans, for instance, aspects of language processing occur predominantly in the left hemisphere. Brain lateralization is thought to increase cognitive performance, whereby specialization of one hemisphere leaves the other free to perform different tasks. Compromised brain asymmetries have been linked to several neuropathologies including schizophrenia, autism, and neuronal degenerative diseases. Yet despite the prevalence and importance of nervous system asymmetries, our knowledge of the mechanisms that underlie the development and functional consequences of asymmetry is far from complete.
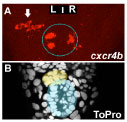
The best-described neuroanatomical asymmetries in vertebrates are in the epithalamus, a dorsally positioned structure in the diencephalic forebrain. Asymmetries consist of differences in size, neuronal organisation, neurochemistry, connectivity and functional properties of neurons. In recent years, we and others have established the zebrafish as a model to study the genetic and developmental mechanisms underlying the establishment of asymmetry. Our studies are now addressing how asymmetry is encoded within circuitry and are beginning to address the behavioural consequences of asymmetric circuitry.
wellcome trust film about our research in collaboration with the Bianco Lab into brain asymmetry
RELATED PUBLICATION SUMMARIES
ASYMMETRIC BRAIN PUBLICATIONS
Ingrid Lekk , Véronique Duboc, Ana Faro, Stephanos Nicolaou, Patrick Blader, Stephen W Wilson.
Sox1a mediates the ability of the parapineal to impart habenular left-right asymmetry.
eLife 2019;8:e47376 DOI: 10.7554/eLife.47376
Roussigné M, Wei L, Tsingos E, Kuchling F, Alkobtawi M, Tsalavouta M, Wittbrodt J, Carl M, Blader P, Wilson SW. (2018)
Left/right asymmetric collective migration of parapineal cells is mediated by focal FGF signaling activity in leading cells.
Proc Natl Acad Sci U S A. pii: 201812016. doi: 10.1073/pnas.1812016115.
Turner, K. J., Hawkins, T. A., Yáñez, J., Anadón, R., Wilson, S. W., & Folgueira, M (2016)
Afferent Connectivity of the Zebrafish Habenulae
Front Neural Circuits. 2016 Apr 26;10:30. doi: 10.3389/fncir.2016.00030. eCollection 2016.
Dreosti E, Vendrell Llopis N, Carl M, Yaksi E, Wilson SW. (2014)
Left-Right Asymmetry Is Required for the Habenulae to Respond to Both Visual and Olfactory Stimuli.
Curr Biol. 2014 Feb 5. pii: S0960-9822(14)00017-7. doi: 10.1016/j.cub.2014.01.016.
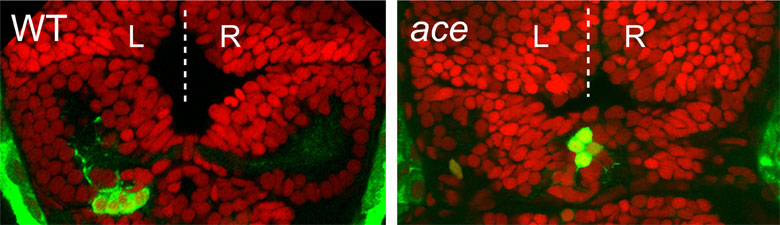
Hüsken U, Stickney HL, Gestri G, Bianco IH, Faro A, Young RM, Roussigne M, Hawkins TA, Beretta CA, Brinkmann I, Paolini A, Jacinto R, Albadri S, Dreosti E, Tsalavouta M, Schwarz Q, Cavodeassi F, Barth AK, Wen L, Zhang B, Blader P, Yaksi E, Poggi L, Zigman M, Lin S, Wilson SW, Carl M. (2014)
Tcf7l2 is required for left-right asymmetric differentiation of habenular neurons.
Curr Biol. 2014 Oct 6;24(19):2217-27. doi: 10.1016/j.cub.2014.08.006.
Roussigne, Myriam; Blader, Patrick; Wilson, Stephen W (2012)
Breaking symmetry: The zebrafish as a model for understanding left-right asymmetry in the developing brain
Devel Neurobio, 72: 269-281. https://doi.org/10.1002/dneu.20885
Bianco IH, Wilson SW. (2012)
Encoding asymmetry within neural circuits.
Nat Rev Neurosci. 2012 Dec;13(12):832-43.
Roussigné M, Bianco I.H, Wilson S.W, Blader P. (2009)
Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei
Development 136:1549-1557.
Regan JC, Concha ML, Roussigne M, Russell C, Wilson SW (2009)
An Fgf8-dependent bistable cell migratory event establishes CNS asymmetry.
Neuron 61:27-34
Bianco,I.H.,Wilson,S.W. (2009)
The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain.
Philosophical Transactions of the Royal Society B:Biological Sciences
Bianco IH, Carl M, Russell C, Clarke JD, Wilson SW. (2008)
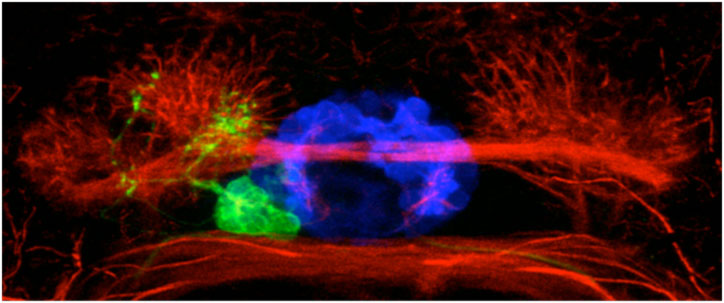
Brain asymmetry is encoded at the level of axon terminal morphology.
Neural Development 3:9-27
Carl, M., Bianco, H.B., Bajoghli, B., Aghaallaei, N., Czerny, T., and Wilson, S.W. (2007)
Wnt/Axin1/b-catenin Signaling Regulates Asymmetric Nodal Activation, Elaboration, and Concordance of CNS Asymmetries.
Neuron 55:393-405
Aizawa,H., Bianco,I.H., Hamaoka,T., Miyashita,T., Uemura,O., Concha,M.L., Russell,C., Wilson,S.W., Okamoto,H. (2005)
Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus.
Current Biology 15:238-243
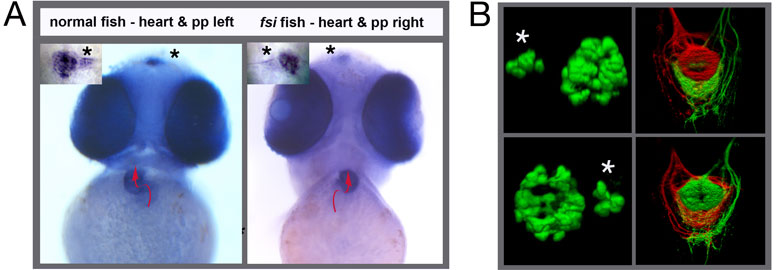
Barth,A., Miklosi,A., Watkins,J., Bianco,I.H., Wilson,S.W., Andrew,R.J. (2005) fsi Zebrafish Show Concordant Reversal of Laterality of Viscera, Neuroanatomy, and a Subset of Behavioral Responses. Current Biology 15:844-850
Concha,M. L., Russell,C., Regan,J. C., Tawk,M., Sidi,S., Gilmour,D. T., Kapsimali,M., Sumoy,L., Goldstone,K., Amaya,E., Kimelman,D., Nicolson,T., Grunder,S., Gomperts,M., Clarke,J. D., Wilson,S. W. (2003)
Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality
Neuron 39:423-438
Cau,E.,Wilson,S.W. (2003)
Ash1a and Neurogenin1 function downstream of Floating head to regulate epiphysial neurogenesis
Development 130:2455-2466
Concha,M.L.,Wilson,S.W. (2001)
Asymmetry in the epithalamus of vertebrates
Journal of Anatomy 199:63-84
Concha,M.L., Burdine,R.D., Russell,C., Schier,A.F., Wilson, S. W. (2000)
A Nodal signaling pathway regulates ythe laterality of neuroanatomical asymmetries in the zebrafish forebrain.
Neuron 28:399-409