Jenny Regan and Steve Wilson
Original paper reference:
An Fgf8-dependent bi-stable cell migratory event establishes CNS asymmetry
Left-right asymmetry is a universal feature of the central nervous system (CNS) and is fundamental to proper brain function. In this study, we sought to answer a question about which virtually nothing was known: "How is symmetry broken in the vertebrate brain?"
The zebrafish brain shows differences between left and right sides in terms of structure, organization and connectivity of nerve cells (neurons). These features have helped to make the zebrafish a focus for studies of brain asymmetry. We have previously shown that the consistent development of brain asymmetries in one direction (laterality or handedness) is dependent on left-sided activity of a Nodal-family signalling protein. Crucially, if Nodal signalling occurs on both sides of the brain or is absent, brain asymmetries still develop, but are randomised, such that normal brain laterality and reversed brain laterality are equally likely outcomes. Therefore, whilst consistent laterality relies on Nodal signalling, development of an asymmetric brain per se does not, and must be dependent on other signals. To uncover the signalling pathways required to break symmetry in the brain, we looked for lines of zebrafish carrying genetic mutations that prevent the development of brain asymmetry.
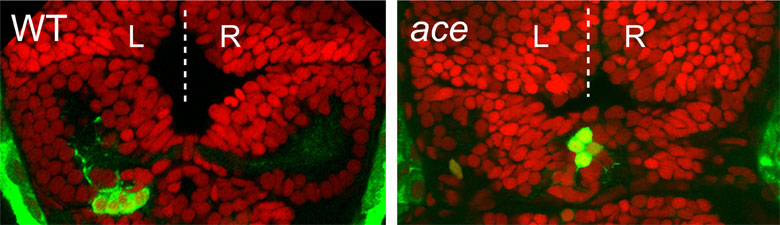
Images of brains of normal (wild-type, WT left) and ace/fgf8 mutant (right) zebrafish in which all cells are labelled with a red nuclear marker (TOPRO-3) and parapineal neurons with an additional green marker (green fluorescent protein). The parapineal neurons are on the left in the wild-type brain but stuck at the middle in the fgf8 mutant.
We discovered that fish carrying a mutation in the fgf8 gene, called acerebellar (ace), have symmetric brains. Although all relevant brain structures are specified in the mutant, they are unable to develop asymmetrically. This is first evident in the failure of a small group of neural cells, the parapineal, to migrate in a stereotypical leftward arc away from the midline. This leftward migration normally initiates a cascade of events that leads to elaboration of brain asymmetries and culminates in the establishment of asymmetric brain circuits. We found that in ace embryos, the parapineal remains at the midline and never migrates, and later-developing brain structures remain symmetric.
When we looked to see where the fgf8 gene is expressed in normal embryos, surprisingly, we found it on both sides of the brain adjacent to the parapineal, as are some genes turned on in response to Fgf signalling. Supporting the idea that Fgf signals act upon the parapineal, several genes functioning in the Fgf-pathway, including the Fgf-receptor FgfR4, are expressed specifically in this structure.
In order to determine whether signalling by Fgf8 is required for the parapineal nucleus to move leftward from the midline, we provided ace brains with a localised source of Fgf8. This was able to rescue the migration of the parapineal nucleus in ace mutants, however, migration was usually to the left, irrespective of the location of the source of Fgf8. This led us to suspect that another signal acts together with Fgf8 to influence the direction of migration. Indeed, we found that the leftward bias in Fgf-dependent migration is due to left-sided Nodal signalling. In situations where the strong Nodal bias is removed, the Fgf8 source can determine the direction of brain laterality, possibly by acting as an attractant to parapineal cells.
This and other data allowed us to produce a model for generation of brain asymmetry, where left and right sides of the brain compete to attract the parapineal via Fgf8-signalling, initiating a cascade of asymmetric development on the winning side. In normal brains (Panel A below), Nodal signalling strongly biases Fgf-dependent migration to the left. However, Nodal in the absence of Fgf8 is not sufficient to promote migration (Panel C). If Nodal is taken away, the side of the brain that wins the competition is probably that which has stochastically slightly higher levels of Fgf8 (Panel B). Indeed, if we experimentally provide Fgf8 on one side in such situations, that side that wins the competition. The study shows that the combined action of Fgf and Nodal signals ensures the establishment of brain asymmetries with consistent laterality, and suggests that mechanisms to generate asymmetry and direct laterality can be uncoupled and may have evolved sequentially.
Schematic representing Fgf8 and Nodal signalling (top) and resulting brain asymmetry (bottom) in normal (wild-type) embryos (A), in embryos with unbiased Nodal signals (B) and in fgf8 mutants (ace, C). Lh, left habenula; Rh, right habenula. The habenulae are paired structures that produce Fgf8 signals and elaborate asymmetries themselves in response to parapineal migration.
For further reporting and discussion of this work
If you would like to read more about our asymmetry research, please visit our asymmetry research web pages. If you have any more questions about this work, please contact Jenny or Steve Wilson
Our work on this project was primarily funded by the Wellcome Trust