Welcome to Matthias who joins the Bianco lab from the Wellcome Optical Biology PhD programme.

Welcome to Matthias who joins the Bianco lab from the Wellcome Optical Biology PhD programme.
First Person is a series of interviews with the first authors of a selection of papers published in Disease Models & Mechanisms, helping early-career researchers promote themselves alongside their papers. Karin Tuschl is first author on ‘ Loss of slc39a14 causes simultaneous manganese hypersensitivity and deficiency in zebrafish’, published in DMM.
Read the interview with Karin about her work investigating the role of manganese in brain physiology and disease.
First Person-Karin Tuschl
Dis Model Mech (2022) 15 (6): dmm049641.
“Loss of slc39a14 causes simultaneous manganese hypersensitivity and deficiency in zebrafish”
Full paper in Disease Models and Mechanisms: https://journals.biologists.com/dmm/article/doi/10.1242/dmm.044594/275312/

Zebrafish brain showing changes in neuronal activity upon manganese neurotoxicity: diminished activity in green, increased activity in magenta, scale bar 100µm (Credit to Chintan Trivedi).
Dysregulation of the metal manganese in the brain underlies movement abnormalities in a rare inherited disorder of manganese transport leading to disability in early childhood.
Manganese is an essential trace metal present in our diet that is required for normal brain function. However, excess manganese is toxic to the brain, particularly to regions required for movement control. Therefore, it is crucial that the body tightly regulates manganese levels.
Dr Tuschl in collaboration with other UCL groups has recently identified an inherited disorder caused by abnormalities in the gene SLC39A14 that is required for manganese transport across the cell. Impaired control of the body's manganese load results in manganese accumulation in the brain. Affected children suffer from a disabling movement disorder and often become wheelchair bound within the first years of life. Current treatment options are limited and burdensome. Therefore, there is a real need to better understand the disease mechanisms in order to identify new treatment targets and strategies.
Using a zebrafish model of this manganese transport disorder this study has identified how manganese regulation is impaired in the brain leading to both toxic effects of manganese as well as manganese deficiency. Subsequently, this impacts on the regulation of calcium that is required for normal neuronal function. These changes are associated with impaired neuronal activity in the brain of zebrafish and reduced swimming behaviour.
The Wilson Lab in collaboration with Monica Folgueira from the University of A Coruña have published a paper focussed on the development of the telencephalon. We had a lot of help from some other Wilson Lab alumni: Pedro, Leo Valdivia and Isaac Bianco.
This paper forms part of a Research topic for the journal Frontiers in Neuroanatomy: Teleostean Forebrain Organization and Evolution: Links to Behaviour and Ecological Niche
Check out our preprint on biorxiv

Cachd1 is a novel Wnt pathway component that bridges FZD and LRP6 co-receptors. Asymmetric modulation of Wnt signalling by Cachd1 leads to lateralisation of Hb neurons by altering timing of neurogenesis and probabalistic selection between lateralised neuronal fates.
This was a fantastic collaborative effort between the Wilson Lab and the Yvonne Jones, Raible and Li Labs and Gavin J Wright. With four joint lead authors; Gareth Powell, Yuguang Zhao, Ana Faro & Heather Stickney.
Read a lay summary of this publication here!
The Bianco lab is looking for postdocs to join a Wellcome funded project examining how circuits integrate sensory and internal state information to select behavioural programmes and how sequences of motor actions are flexibly selected and tuned to accomplish behavioural goals. The lab uses a variety of approaches including functional calcium imaging, naturalistic behavioural assays, multiphoton optogenetics, circuit tracing and computational modelling.
Please contact Isaac to find out more and apply before 6 June 2022: bit.ly/3M1rwsT
24th-28th October 10:00-16:00
***Applications for the 2022 work experience are now being accepted. ***
Please note you must be in Sixth Form at a UK school at the time of application and at the time of the work experience placement.
***SUBMISSION DEADLINE EXTENSION TILL 29th May 2022***
Please note applications will not be accepted beyond this date.
For more information about the programme click the button below.
The Tuschl lab secure further funding from:
GOSH BRC Junior Faculty Consumable Support (£10,000): to develop antisense oligonucleotide treatment for manganese neurotoxicity.
&
UCL Therapeutic Innovation Networks (TINs) Pilot Data Scheme – Small Molecules (£10,000): to develop novel chelators for the treatment of manganese neurotoxicity in collaboration with the group of Prof John Spencer and Dr George Lostakis at the University of Sussex.


Elena Dragomir has been awarded a four-year Sir Henry Wellcome Fellowship to work on the role of the asymmetric habenular nuclei in state-dependent modulation of behavioural preference. Elena’s research will span between Steve’s and Isaac Bianco’s groups with input on modelling approaches from James Fitzgerald at Janelia Research Campus.
The Wong Lab is looking to recruit one Postdoctoral Research Fellow. The position is funded by the Wellcome Trust and Royal Society for 3 years. Please apply if you are interested in morphogenesis, cell migration, signalling and doing some great imaging and fun science. Contact Mie (mie.wong@ucl.ac.uk) if you are interested to find out more.
More details can be found here:
https://www.wongmorphogenesislab.com/
UCL vacancy reference number: 1881595
The application deadline is 2 Feb 2022.
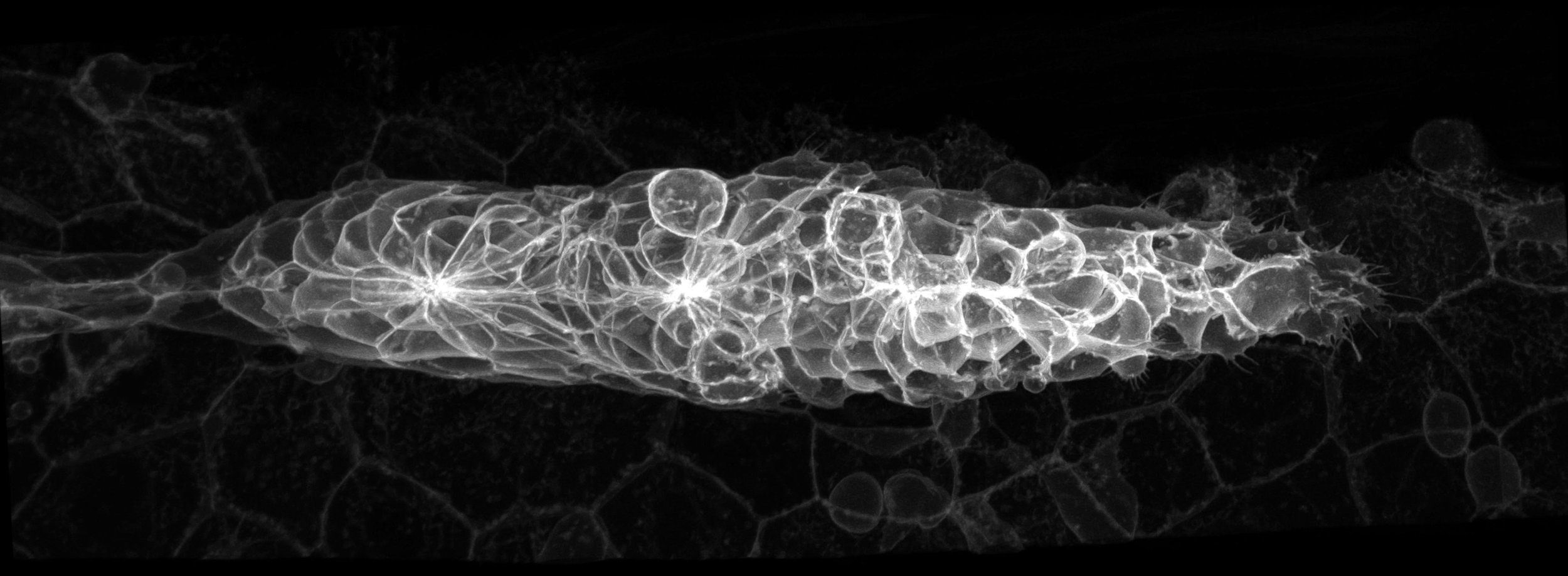
The zebrafish lateral line primordium.

Mie Wong has joined the Fish Floor and the Department of Cell and Developmental Biology, UCL, as a Wellcome Trust and Royal Society Sir Henry Dale Fellow. She was previously at the University of Zurich, Switzerland.
Mie's lab studies how moving cells shape the developing embryo. The lab is especially interested in understanding the interplay between extrinsic signals and collective cell behaviours in establishing and maintaining the directionality of cell migration during development. The primary model used in the lab is a system of sensory organs called the lateral line. The Wong Lab employs interdisciplinary approaches including genetic, chemical and optical manipulations combined with advanced quantitative imaging, mass spectrometry and mathematical modelling.
Click here (https://www.wongmorphogenesislab.com/) to find out more about the lab.

Karin Tuschl and the team AccessBrain win the UCL ACCELERATE Innovation Team Challenge and £10,000 to develop a human iPSC-derived 3D blood brain barrier model (BBB) for assessment of BBB permeability of drug candidates and excipients.

First Floor Zebrafish Labs were awarded GOLD in the Laboratory Efficiency Assessment Framework (LEAF) for our efforts to improve sustainability and efficiency in all areas of laboratory practice. Check out the Zebrafish Green Lab section of our homepage to see what practices we have put in place and get some ideas on how to make your lab greener. Keep up the good work!


Interested in whole brain activity and neurochemistry mapping, in vivo calcium imaging of neuronal activity, single-cell transcriptomics, behavioural analysis and genome editing? Then join us at ZEBRAFISH UCL to study the role of manganese in health and disease.
Details here: tinyurl.com/3yweh4cn
UCL vacancy reference number: 1876901
We all enjoyed another excellent UCL Neuroscience Symposium, which featured fantastic talks by our own Charlie and Asaph.


Advances in CRISPR/Cas9 technologies allow researchers to rapidly test the function of genes by knocking them out in injected embryos, reducing the number of animals and time needed to generate stable mutant lines (Kroll et al. 2021 eLife 10: e59683; https://doi.org/10.7554/eLife.59683). However, determining the efficacy of CRISPR/Cas9 ribonucleoproteins (RNPs) often requires access to expensive and specialised equipment (e.g. deep sequencing or high resolution melting analysis) or restricts the potential target sequences (e.g. restriction fragment length polymorphism analysis).
Recently, we adapted and piloted a method of suppression PCR called headloop PCR (Rand et al. 2005 Nucleic Acids Research 33: e127, https://doi.org/10.1093/nar/gni120; Kroll et al. 2021 eLife: 10:e59683). This proved to be a sensitive and robust way to identify non-functioning and low efficiency guide RNP complexes. We’ve observed that headloop PCR is sensitive to a wide range of mutations, including small compound indels, and those at low copy number. Its performance correlates well with deep sequencing analysis. It is simple, cheap and easily adoptable, requiring only basic molecular biology reagents and equipment, and is flexible, with no constraints on guide RNA sequences.
Importantly, we believe it could also be used to support the aims of the 3Rs project – to help reduce the use of animals in retesting negative results and in the generation of stable mutant lines. Having identified genes of interest for further study, researchers can use the same validated RNPs to create inherited stable mutations. Unfortunately, most methods only indicate that mutations have been made in the respective targets; determining the nature of those mutations can be difficult from ambiguous sequencing data. Because headloop PCR is effectively allele-specific, the reaction products can be sequenced to determine the nature of the inherited lesions directly.
For further details about headloop PCR and its use in testing CRISPR/Cas9 guides please see our article at eLife: Kroll et al. 2021 . More detailed information and resources for headloop PCR are available at our Protocols page.

The Bianco lab is looking to recruit one/two postdoctoral research fellows.
Positions are Wellcome funded for 4 years. Experience with custom design of optical systems or electrophysiology would be especially great, but most important is enthusiasm to understand the circuit basis of behaviour.
NOTE: We have extended the deadline for applications so you can now apply up until 30 NOVEMBER. Contact Isaac if you’re interested to find out more.

Check out Francois Kroll's preprint describing simple CRISPR/Cas9 methods to knockout genes for behavioural studies.
https://www.biorxiv.org/content/10.1101/2020.06.04.133462v1

We’re delighted to announce that the Wellcome Trust are generously funding the Bianco lab for the next five years.
We will be using fast calcium imaging, electrophysiology and holographic optogenetic stimulation to figure out how zebrafish command and pattern their hunting sequences.
We’ll be advertising postdoc positions soon, so please contact Isaac if you’re interested.
As anyone who does 2-photon imaging in behaving animals will know, motion artifacts are a pain the neck and can be difficult to correct. We were lucky enough to contribute to a fantastic project in Angus Silver’s lab, which led to technology enabling real-time motion correction in 3D. As Joanna showed, it works beautifully in larval zebrafish!
